Interim Report
Marsh Ecology Research Program
Project Title: Patterns of ontogenetic shifts in nekton habitat use along a marsh coenocline: Atlantic silverside case study.
Project Leader:
Rodney Rountree, Ph.D., Adjunct Assistant Professor, Dept. Natural Resources Conservation, UMASS-Amherst, 48 Oregon Rd., Mashpee, MA 02649, email: rrountree@capecod.net
Co-Investigators
Francis Juanes, Ph.D., Assistant Professor, Dept. Natural Resources, University of Massachusetts at Amherst, Amherst, MA 01003-4210, Ph: email: juanes@forwild.umass.edu
Abstract
A growing body of evidence suggest that communities of marsh nekton are stratified along a depth gradient partly in response to the influence of tidal and diel changes in physical conditions. In addition, ontogenetic shifts in habitat use along this gradient are key to our understanding of marsh function and the process of trophic relay, yet have rarely been specifically addressed. The goal of this project is to develop sampling methodologies and collect preliminary data to test the hypothesis that nekton faunal assemblages, species densities, and ontogenetic stages of Atlantic silversides and other species, are stratified along a marsh creek-to-bay coenocline (i.e., the marsh gradient) partly in response to diel and tidal cycles in environmental gradients. Preliminary results include: 1) successful development and use of a new seine sampling technique that provides density estimates of nekton in marsh creek and shallow bay environments, 2) we obtained sufficient preliminary data (69 seine samples) to compare the effectiveness of this new technique against a more conventional standardized seining method, 3) we obtained preliminary data on the diel and tidal changes in temperature along the marsh gradient, 4) we obtained preliminary data illustrating strong diel changes in nekton density distributions and faunal assemblages along the marsh gradient, and 5) we obtained preliminary data suggesting strong ontogenetic shifts in density distributions along the marsh gradient, and of strong interactions with diel period suggesting ontogenetic diel migration patterns.
Introduction
Although numerous recent studies have suggested that marsh creeks are important nursery habitats for marine fishes and invertebrates (Bozeman & Dean 1980, Weinstein et al. 1980, Currin et al. 1984, Weinstein 1984, Weinstein et al. 1984, Cowan & Birdsong 1985, Teal 1985, Blaber 1986, Sogard & Able 1991, Rountree & Able 1992a), little is known of factors which regulate marsh creek communities. In tidal marsh creeks gradients in physical conditions can form along the mouth-to-headwater creek gradient (hereafter referred to as the marsh gradient, Fig. 1) as a result of interactions between the influences of the adjacent bay waters, atmospheric conditions, and terrestrial conditions (Hackney et al. 1976, Daiber 1977). Similar gradients can occur along the creek cross-section (Kneib 1984). Generally, because of the decreasing depth and water volume, and increasing distance from the bay, physical conditions are increasingly influenced by atmospheric and land conditions moving up the creek, while bay conditions have an increasing influence moving down the creek. Additionally, bay conditions have their greatest influence at high tide and atmospheric/land conditions at low tide (Hackney et al. 1976, Daiber 1977). Over a diel and tidal cycle, conditions become increasingly variable (with greater maxima and minima values) moving up the creek gradient, being maximal in intertidal areas, but become more stable moving down the creek, being minimal in the bay. Therefore, in addition to the physical gradient formed along the creek at any given time, a gradient in environmental variability along the creek is formed over the diel and tidal cycle.
A growing body of evidence suggests that communities of marsh nekton are stratified along a depth gradient partly in response to the influence of tidal and diel changes in physical conditions (Rountree 1992). It is hypothesized that nekton species each have a basic distribution along the creek gradient resulting from its response to these physical gradients. However, species interactions such as competition, risk of predation, etc., may also influence marsh creek species distributions (McIvor & Odum 1988, Shirley et al. 1990). Mechanisms regulating the distributions of animals in tidal creeks, therefore, parallel those regulating other intertidal habitats, being controlled by balances between physiological tolerances/preferences, predation pressures, and competitive interactions (Connell 1961, Kneib 1984). However, the highly motile fauna of marsh creeks have the option of moving between areas in response to changes in physical conditions, in contrast to sessile communities (Connell, 1961). Therefore, distributions of some species may shift up and down the creek gradient in response to cyclical tidal and diel changes in physical and biological gradients along the creek gradient.
These hypotheses are supported by some previous studies which either provide evidence of community differences among marsh habitats (e.g., subtidal creek, intertidal marsh surface, and marsh ponds, Subrahmanyam & Drake 1975, Subrahmanyam & Coultas 1980, McIvor & Odum1988, Able et al. 1996), or provide evidence of community changes between locations within creeks (Hackney et al. 1976, Weinstein 1979, Weinstein & Brooks 1983, Rozas & Hackney 1984,Smith et al. 1984, Rozas & Odum 1987, McIvor & Odum 1988, Hettler 1989). The influence of location within a creek on community structure has variously been described as occurring along a mouth-to-headwater creek gradient (Hackney et al. 1976), along a marsh creek coenocline (Weinstein 1979, Weinstein & Brooks 1983, Smith et al. 1984), or along a marsh creek order gradient (Rozas & Odum 1987, Hettler 1989). Hackney et al. (1976) and Hackney (1977) similarly found that species abundance and assemblages changed along a mouth-to-headwater creek gradient in response to tidal and diel gradients in physical conditions.
Although density and size class structure data will be obtained for all nekton, the major focus of this pilot study will be the Atlantic silverside, Menidia menidia. Atlantic silversides were chosen as the target because they are arguably the most abundant marine transient utilizing saltmarsh habitats in New Jersey (Rountree and Able 1992a), have been suggested to be key vectors of trophic relay from the salt marsh to coastal waters (Conover and Ross 1982, Rountree and Able 1992a, Deegan et al. in press), and appear to exhibit ontogenetic shifts in habitat use patterns (Rountree and Able 1992a, 1993).
It has been hypothesized that extensive nocturnal migrations of Atlantic silversides into shallow marsh habitats results from predator avoidance behavior, and that there is an ontogenetic shift in this behavior with smaller YOY tending to remain within the marsh, and larger individuals migrating between open water feeding grounds and shallow water predator refugia (Rountree and Able 1993). The hypothesized predator refuge function of marshes for Atlantic silversides is strengthened by recent findings of nocturnal migrations of large predators, including bluefish and weakfish into shallow bay shoals adjacent to marsh creeks (Rountree and Able 1997). Additionally, Atlantic silversides are known to be a vital forage species for YOY summer flounder (Rountree and Able 1992b) and other marine transients that utilize the salt marsh as a nursery. In fact, it has been reported that seven of the ten dominant marine transients utilizing New Jersey marshes may match their habitat use with the timing of Atlantic silversides' shift from marsh creek to open bay waters (Rountree and Able 1992a). These patterns suggest that the importance of Atlantic silversides to the trophic relay of energy from the marsh is more complex than previously thought, and that the species may transfer energy into the marsh from the bay, rather than export energy because they are known to feed primarily on zooplankton and exclusively during the day.
The goal of this project is to develop sampling methodologies and collect preliminary data to test the hypothesis that nekton faunal assemblages, species densities, and ontogenetic stages of Atlantic silversides and other species, are stratified along a marsh creek-to-bay coenocline (i.e., the marsh gradient) partly in response to diel and tidal cycles in environmental gradients. Diel, tidal and creek gradients in physical conditions along a marsh creek habitat coenocline (Fig. 1) will be examined and correlated with nekton density and size class structure to determine marsh function for target species. Specific goals include: 1) Develop a quantitative seine methodology that will allow a comparison of nekton densities at various locations along the marsh gradient, including open bay waters, 2) Quantify densities of Atlantic silversides and other nekton by ontogenetic stage along the marsh gradient, and 3) Quantify tidal and diel cycles in water temperature along the marsh gradient and correlate with ontogenetic shifts in habitats.
Methods and Preliminary Results
The study was conducted in marsh creek and adjacent shallow bay shoal habitats in a polyhaline (22-33‰) section of the Little Egg Harbor estuary located in southern New Jersey (Fig. 2). Foxboro Creek is a shallow subtidal marsh creek (maximum depth 0.5 m at low tide and 1.1 m at high tide), with a shallow sill (<30 cm deep at low tide) at its mouth. Foxboro Creek has a mud substrate and empties onto extensive shoal areas (<2.0 m at high tide) bordering the relatively deep Marshelder Channel (approx. 4-9 m at high tide; Fig. 2). The creek is a blind cul-de-sacs, approximately 1 km long, that receives freshwater only through local run-off. Foxboro Creek was chosen as the sampling site because it has been previously extensively studied (Rountree and Able 1992a,b, 1993,1996,1997) and was ideal for the purposes of this study. Specific sampling sites along the marsh gradient were randomly chosen within each of three location strata: Upper Creek, Lower Creek, and bay (Fig. 2). The Upper and Lower creek strata were arbitrarily defined by the major fork in the creek (located at about 246 m from the creek mouth), while the bay strata was demarcated from the creek strata by the creek mouth. To facilitate the stratified random sampling design, the subtidal portion of the creek was measured and reference poles were set out at various intervals along the creek (Fig. 3). Prior to sampling, the location of the samples within each stratum would be randomly determined and found by measuring the appropriate distance from the nearest reference pole. For example, if a sampling location of 154 m was randomly selected, then it would be found by measuring 4 m up creek from the 150-m reference pole. Using this procedure we were able to rapidly and accurately locate the sampling sites.
Environmental gradients
Low tide depth profiles of the creek were measured at various locations along its length (Fig. 4) to correlate creek morphology parameters with nekton densities. Low tide depth profiles were also necessary for calculations of nekton densities at specific sites. A comparison of the cross-sectional depth profile of Foxboro Creek at one site over several consecutive tides illustrates the importance of tidal variation in depth to density estimation (Fig. 5). While the creek was 14 m wide at this location during two consecutive day tides, its width more than doubled to 33 m during the night high tide (asymmetrical day and night tide heights are a well know phenomenon in this geographic region). It is also important to note that the creek width dropped from 8 m to 6 m during the time it took to collect the seine samples despite our effort to sample as close to low tide as possible. Such changes probably have strong impacts on the tidal concentration effect on nekton densities and require further investigation.
Continuous recording of water temperatures were made at various locations along the creek length during both August 2-6 and August 23-27 sampling trials using miniature electronic temperature data loggers (StowAway TidbiT, manufactured by Onset Computer Corporation). We observed dramatic differences in the temporal (diel and tidal) temperature patterns among creek locations, with low diel and tidal variation in bay locations and increasing temporal variation moving up the creek axis (Fig. 6 and 7). In creek locations water temperatures rose 2-3 C within a few hours after initial deployment (about 2 p.m.), before gradually declining 6 C over the next 12 hours during the August 2-6 sampling (Fig. 6). The following morning temperature again began to rise to a peak in the early evening before declining through the night. In contrast, temperatures remained stable in the bay locations (Fig. 6). This pattern suggests a strong interaction between tidal stage and diel period. We expected to see a gradual rise in temperatures through mid-day, followed by a gradual decline in temperature through the night. But because the tidal and diel cycles were out of phase, the resulting water temperature patterns were more complex. For this reason, we decided to measure ambient air temperatures as well as water temperature during the August 23-27 sampling (Fig. 8). Note that the air temperatures at both the -125 m and 494 m locations exhibit the same simple pattern of warm day and cool night temperatures. Water temperature mirrored air temperatures in the upper creek locations, but the diel effect was gradually dampened towards the bay locations. However, a slight rise in temperatures occurred in the evening hours (around 1130 PM) corresponding to low tide suggesting outwelling of a slug of warm waters from the marsh. This data suggests that diel and tidal cycles in temperatures vary greatly along the marsh gradient, and probably have an important role in regulating marsh use patterns by nekton. Resident nekton must be adapted to cope with sometimes-strong diel and tidal changes in water temperatures, the magnitude of which is a function of location along the marsh gradient (as much as 1-2 C/h). Just as importantly, because of the interaction between tidal and diel effects, the pattern of these changes themselves can fluctuate daily, making adaptation more difficult (i.e., a consistent diel cycle in water temperatures where temperature rise to a peak during the day and decline to a minimum at night can be more readily adapted to, than a variable diel cycle).
Standardized Seine Sampling
A new type of standardized seine methodology was developed to quantify nekton densities along the marsh gradient at low tide (Fig. 8). We used a stratified random sampling design with replication. The length of the subtidal portion of Foxboro Creek was measured and three location strata were arbitrarily defined: Upper Creek, Lower Creek, and Bay as described above. The sampling design called for sampling within two adjacent 5-meter sections of the gradient within each stratum. We attempted to achieve this by suspending block nets above the water line at high tide and dropping them with a remote trigger line at low tide. In the creeks this was done by suspending three block nets between the creek banks at 5-m intervals. In the bay, we suspended a single 36-m long block net between six poles forming a 5-m x 13-m rectangle. As in the creeks, the nets could be dropped simultaneously using long trigger lines. Unfortunately, although we successful in developing this large drop-net technique, it required permanent supporting poles (i.e., to support the full weight of the heavily chained nets, we had to use steel poles that could not easily be removed after sampling). Because our sampling design necessitates random sampling along the creek axis, and avoidance of faunal depletion and repeated sampling effects, we had to abandon this technique. We were able to develop alternative sampling methods, but had to settle for slight differences in metrologies between bay and creek strata.
Bay sampling-Temporary support pools were set out to form a 5-m by 13-m rectangular sampling area. Then the 36-m long block net was walked along the perimeter of the rectangular area and tied off to the support poles at low tide to enclose a 65-m2 area. Unfortunately, because we felt that this method of manually deploying the block net caused too much potential disturbance to the nekton, we could not sample immediately adjacent 5-m sections of the gradient in the bay strata. To sample in the bay strata, therefore, we randomly chose two different locations to use as within-treatment replications. Once the block net was in place we used a 7.6-m (25 ft) long seine (1.2 m deep, 3.2mm sq. mesh) with attached tunnel and live car to conduct standardized seines both within and outside of the block net for comparison. A 5-m long restriction line was tied between the seine poles to insure a standard 5-m wide sweep path. From 1 to 3 seine hauls were made 5 m away from and parallel to the block net to obtain a comparative 5-m x 13-m sweep area. Then 3 hauls were similarly made inside of the block net.
Creek sampling-Once the specific creek strata sampling sites were randomly chosen as described above, the block nets and support poles were placed in the field at high tide and readied for low tide deployment with a "clothesline" system (Fig. 8). At low tide investigators approached the sampling site on foot keeping at least 10 m from the bank at all times and took up a pull-line in preparation for setting the nets. After a two minute quite period, each of three investigators simultaneously pulled a block nets across the creek. Using this method we were able to simultaneously block off two adjacent 5-m sections of the creek in as little as 15 seconds. The method was surprisingly quite and appeared to cause minimal disturbance to the nekton (far less than even walking along the back would cause, pers. Observ.). Once the block nets were in place, we again used the tunnel seine and live car to sample within and outside of the block nets as was done in the bay strata.
Comparison of seine methods
Although the data has not yet been analyzed our observations suggests that the block net methodology we employed resulting in the higher catch diversity, especially in the bay samples, and higher abundance of many species. For example large fishes such as summer flounder, adult American eels, and juvenile weakfish were captured in the bay block net samples but not in the comparison seine samples. It is our impression too, that larger numbers of juvenile menhaden and silversides were taken in the creek block nets than in the adjacent control seine samples. In addition, there was a non-significant trend for catch size to remain constant among repeated seine hauls for the outside seines, but to decline sharply among repeated hauls within the block nets (Fig. 11). We suspect this resulted from a depletion of the nekton within the block nets (a desired effect, since we are attempting to measure nekton densities), but a continued replacement of fish from the surrounding area in the outside seine samples. Thus, even if catches are equivalent for the first seines in both the block net and outside samples, the block net approach is necessary to estimate actual nekton densities.
Marsh gradient effect
There was a highly significant difference in total catch volume among marsh strata (Fig. 9), which was exaggerated during night samples (Fig. 10). Although the catch data has not yet been fully keypunched and analyzed, a preliminary assessment of the data suggests very strong faunal assemblage differences among the marsh strata (Table 1). Diversity was lowest in the upper creek strata and highest in the bay strata, with larger species such as summer flounder and weakfish being restricted to the bay strata. Our observations suggest that nighttime sampling resulted in dramatically higher catches than during the day in good agreement with a previous study of marsh creek nekton in the area (Rountree and Able 1993). For example we collected over 1600 juvenile menhaden and 27,000 Atlantic silversides from a single night seine sample. That corresponds to about 40 menhaden and 675 Atlantic silversides per m2! These are among the highest fish densities every recorded for any marine habitat. Our observations while processing the samples suggest strong diel, spatial and ontogenetic effects on nekton densities. For example, two species of grass shrimp (Palaemonetes pugio, and P. vulgaris) exhibited differential spatial patterns, but also appear to exhibit strong ontogenetic related diel movement patterns based on changes in size class structure between day and night samples along the marsh gradient.
Bibliography
Able, K.W., D.A. Witting, R.S. McBride, R.A. Rountree, and K.J. Smith. 1996. Fishes of polyhaline estuarine shores in Great Bay-Little Egg Harbor, New Jersey: a case study of seasonal and habitat influences. Chapter 14. In: Nordstrom, K.F., and C.T. Roman (eds.). Estuarine Shore: Evolution, Environments and Human Alterations. John Wiley & Sons, New York. John Wiley & Sons Ltd.
Blaber, S.J.M. (1986). Feeding selectivity of a guild of piscivorous fish in mangrove areas of Northwest Australia. Aust. J. Mar. Freshw. Res. 37: 329-336
Bozeman, E.L., Jr., Dean, J.M. (1980). The abundance of estuarine larval and juvenile fish in a South Carolina intertidal creek. Estuaries 3: 89-97
Connell, J.H. (1961). The influence of interspecific competition and other factors on the distribution of the barnacle Chthamalus stellatus. Ecology 42: 710-723
Conover, D.O., and M.R. Ross. 1982. Patterns in seasonal abundance, growth and biomass of the Atlantic silverside, Menidia menidia, in a New England estuary. Estuaries 5:275-286.
Cowan, J.H., Jr., Birdsong, R.S. (1985). Seasonal occurrence of larval and juvenile fishes in a Virginia Atlantic coast estuary with emphasis on drums (Family Sciaenidae). Estuaries 8(1):48-59
Currin, B.M., Reed, J.P., Miller, J.M. (1984). Growth, production, food consumption, and mortality of juvenile spot and croaker: a comparison of tidal and nontidal nursery areas. Estuaries7(4A): 451-459
Daiber, F.C. (1977). Salt-marsh animals: distributions related to tidal flooding, salinity and vegetation. Chapter 5. Pages 79-108, In: Chapman, V.J. (ed.). Wet Coastal Ecosystems. Elsevier Scientific Publishing Company, Amsterdam
Deegan, L.A., J.E. Hughes and R.A. Rountree. (in press). Salt marsh support of marine transient species: fact or fiction? In: Weinstein, M., and D. Kreeger (eds). Proceedings of the Special International Conference: Concepts and Controversies in Tidal Marsh Ecology, held April 5-9,1998 at Vineland, N.J.
Hackney, C.T. (1977). Energy flux in a tidal creek draining an irregularly flooded Juncus marsh. Ph.D. Dissertation. Mississippi State University, Mississippi State, Mississippi.
Hackney, C.T., Burbanck, W.D., Hackney, O.P. (1976). Biological and physical dynamics of a Georgia tidal creek. Chesapeake Sci. 17(4): 171-280
Harris, R.J. (1985). A Primer of Multivariate Statistics. Academic Press, Inc.
Hettler, W.F., Jr. (1989). Nekton use of regularly-flooded saltmarsh cordgrass habitat in North Carolina, USA. Mar. Ecol. Prog. Ser. 56: 111-118
Kneib, R.T. (1984). Patterns of invertebrate distribution and abundance in the intertidal saltmarsh: causes and questions. Estuaries 7(4A): 392-412
Kneib, R.T. (1998). The role of tidal marshes in the ecology of estuarine nekton. Oceanography and Marine Biology: an Annual Review 35:163-220.
McIvor, C.C., Odum, W.E. (1988). Food, predation risk, and microhabitat selection in a marsh fish assemblage. Ecology 69(5): 1341-1351
Rountree, R.A. 1992. Fish and macroinvertebrate community structure and habitat use patterns in salt marsh creeks of southern New Jersey, with a discussion of marsh carbon export. Ph.D. Dissertation, Rutgers, The State University of New Jersey. New Brunswick. U.M.I.#DAI-9232951. 292 p.
Rountree, R.A., and K.W. Able. 1992a. Fauna of polyhaline subtidal marsh creeks in southern New Jersey: composition, abundance and biomass. Estuaries 15(2):171-185.
Rountree, R.A., and K.W. Able. 1992b. Foraging habits, growth, and temporal patterns of saltmarsh creek habitat use by juvenile summer flounder in New Jersey. Transactions of the American Fisheries Society 121(6):765-776.
Rountree, R.A., and K.W. Able. 1993. Diel variation in decapod crustacean and fish assemblages in New Jersey marsh creeks. Estuarine, Coastal and Shelf Science 37:181-201.
Rountree, R.A., and K.W. Able. 1996. Seasonal abundance, growth and foraging habits of juvenile smooth dogfish, Mustelus canis, in a New Jersey estuary. Fishery Bulletin 94(3):522-534.
Rountree, R.A., and K.W. Able. 1997. Nocturnal fish use of New Jersey marsh creek and adjacent bay habitats. Estuarine, Coastal and Shelf Science 44:703-711.
Rozas, L.P., Hackney, C.T. (1984). Use of oligohaline marshes by fishes and macrofaunal crustaceans in North Carolina. Estuaries 7: 213-224
Rozas, L.P., Odum, W.E. (1987). Use of tidal freshwater marshes by fishes and macrofaunal crustaceans along a marsh stream-order gradient. Estuaries 10(1): 36-43
Shirley, M.A., Hines, A.H., Wolcott, T.G. (1990). Adaptive significance of habitat selection by molting adult blue crabs Callinectes sapidus (Rathbun) within a subestuary of central Chesapeake Bay. J. Exp. Mar. Biol. Ecol. 140: 107-119
Smith, S.M., Hoff, J.G., O'Neil, S.P., Weinstein, M.P. (1984). Community and trophic organization of nekton utilizing shallow marsh habitats, York River, Virginia. Fish. Bull. U.S.82(3): 455-467
Sogard, S.M., Able, K.W. (1991). A comparison of eelgrass, sea lettuce macroalgae, and marsh creeks as habitats for epibenthic fishes and decapods. Estuar. Coast. Shelf Sci. 33(5): 501-520
Subrahmanyam, C.B., Coultas, C.L. (1980). The animal communities in two north Florida, USA saltmarshes: Part III. Seasonal fluctuations of fish and macroinvertebrates. Bull. Mar. Sci. 30:790-818
Subrahmanyam, C.B., Drake, S.H. (1975). Studies on the animal communities in two north Florida salt marshes. I. Fish communities. Bull. of Mar. Sci. 25: 445-465
Teal, J.M. (1985). The role of one salt marsh in coastal productivity. p. 241-258. In: N.L. Chao and W. Kirby-Smith (eds.), Proceedings of the International Symposium on Utilization of Coastal Ecosystems: Planning, pollution and productivity. Universidade do Rio Grande, Brazil
Weinstein, M.P. (1979). Shallow marsh habitats as primary nurseries for fishes and shellfish, Cape Fear River, North Carolina. Fish. Bull. U.S. 77(2): 339-357
Weinstein, M.P. (1984). Comparative ecology of macrofauna of saltmarshes: toward an ecosystem synthesis: epilogue. Estuaries 7(4A): 469-470
Weinstein, M.P., Brooks, H.A. (1983). Comparative ecology of nekton residing in a tidal creek and adjacent seagrass meadow community composition and structure. Mar. Ecol. Prog. Ser.12(1): 15-28
Weinstein, M.P., Scott, L., O'Neil, S.P., Siegfrid, R.C., II., Szedlmayer, S.T. (1984). Population dynamics of spot, Leiostomus xanthurus, in polyhaline tidal creeks of the York River Estuary, Virginia. Estuaries 7(4A): 444-450
Weinstein, M.P., Weiss, S.L., Hodson, R.G., Geny, L.R. (1980). Retention of three taxa of postlarval fishes in an intensively flushed tidal estuary, Cape Fear River, North Carolina. Fish. Bull. U.S. 78(2): 419-436
Table 1. Preliminary list of species collected by marsh gradient strata (location) after completion of 70 % of the sample processing. Present (*), abundant (**), very abundant (***)
|
|
Creek Location Strata |
||
|
Species |
Upper |
Lower |
Bay |
|
Crustaceans |
|
|
|
|
Callinectes sapidus |
* |
** |
* |
|
Crangon septemspinosa |
|
* |
* |
|
Hippolyte pleuracantha |
|
|
* |
|
Libinia dubia |
|
|
* |
|
Pagurus longicarpus |
|
* |
* |
|
Palaemonetes intermedius |
|
* |
|
|
Palaemonetes pugio |
*** |
** |
* |
|
Palaemonetes vulgaris |
** |
*** |
** |
|
Palaemonetes spp. |
*** |
*** |
* |
|
Squilla empusa |
|
|
* |
|
Fishes |
|
|
|
|
Brevoortia tyrannus |
** |
** |
* |
|
Cynoscion regalis |
|
|
* |
|
Cyprinodon variegatus |
** |
* |
|
|
Fundulus heteroclitus |
*** |
** |
* |
|
Fundulus majalis |
* |
* |
|
|
Gobiosoma spp. |
* |
** |
* |
|
Lucania parva |
** |
* |
* |
|
Menidia menidia |
*** |
*** |
* |
|
Mugil cephalus |
|
* |
|
|
Mugil curema |
* |
* |
|
|
Paralichthys dentatus |
|
|
* |
|
Pleuronectes americanus |
|
|
* |
|
Sphoeroides maculatus |
|
|
* |
|
Sphyraena borealis |
|
|
* |
|
Syngnathus fuscus |
|
|
* |
|
Tautoga onitis |
|
|
* |

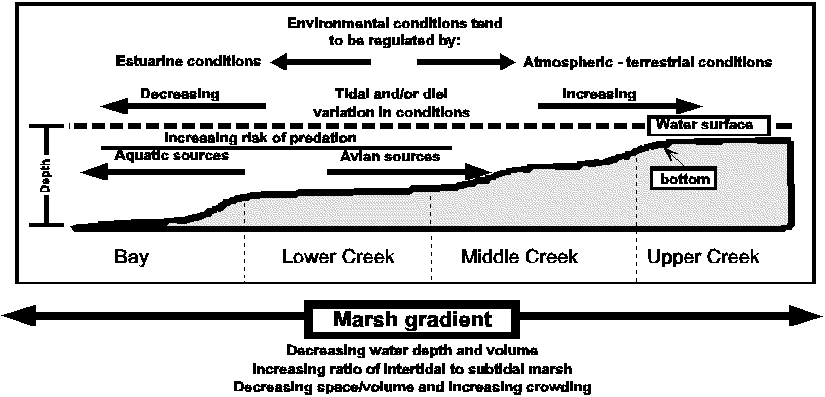
Figure 1. Schematic of hypothetical marsh gradient through a tidal creek to bay coenocline. The gradient is arbitrarily divided into four constituent habitat types making up the coenocline. The marsh habitat gradient in depth, water volume, and ratio of intertidal to subtidal marsh areas, would result in the development of weak to strong horizontal stratification of physical factors such as water temperature. These in turn, together with biotic gradients in factors such as risk of predation, may regulate nekton assemblage and size class distributions along the gradient (adapted from Rountree 1992).
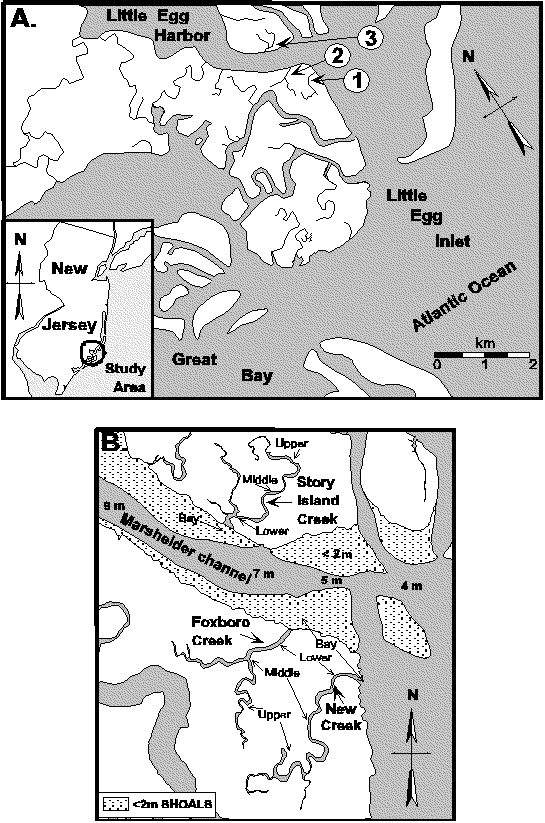
Figure 2. (A) Little Egg Harbor-Great Bay estuary indicating the location the Foxboro Creek study site. (B) Detail of Foxboro Creek indicating approximate location of three habitat zones (Upper Creek, Lower Creek, and Bay).
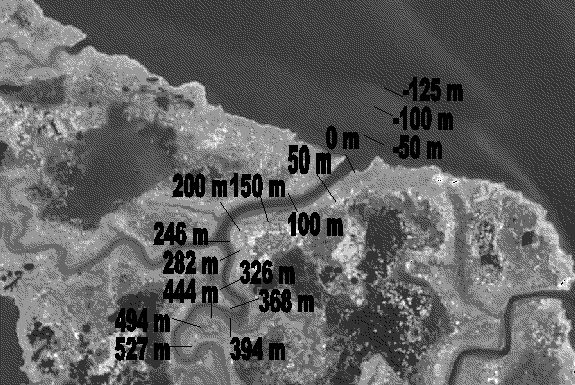
Figure 3. The location of reference markers used to determine sample locations are shown superimposed over an aerial photograph of the Foxboro Creek study site. The location of the markers was measured relative to the creek mouth (0 m). Sampling strata were defined as -1m to -125 m, 0 m to 246 m, and 247 m to 527 m for Bay, Lower Creek, and Upper Creek strata, respectively.
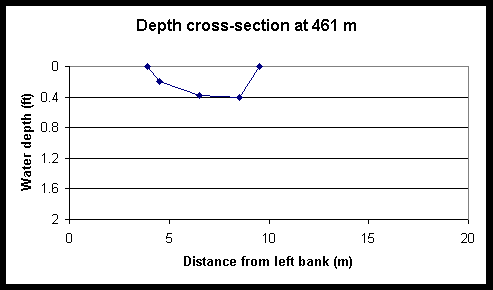
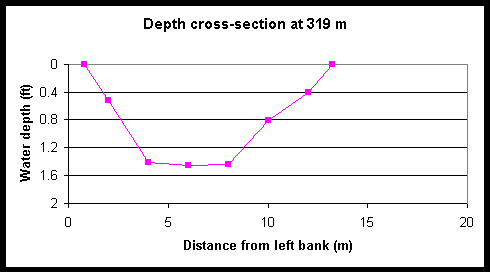
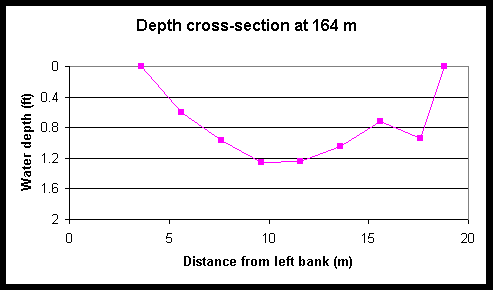

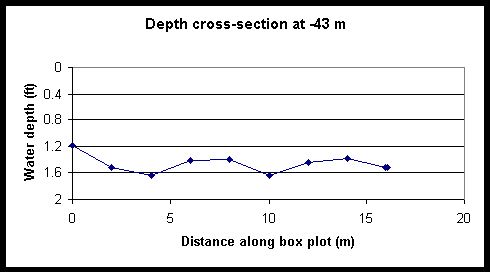
Figure 4. Cross-sectional depth profiles of Foxboro Creek at various distances from the creek mouth.
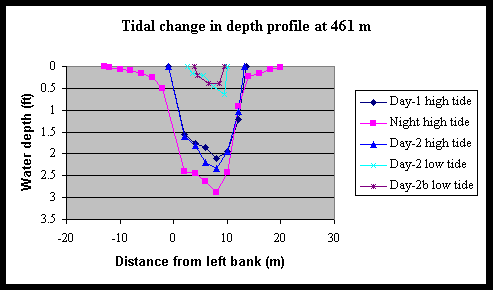
Figure 5. Tidal and diel changes the cross-sectional depth profile of Foxboro Creek at a position located 461-m up-creek of the mouth. The left and right creek banks are located at 0 and 12 m, respectively.
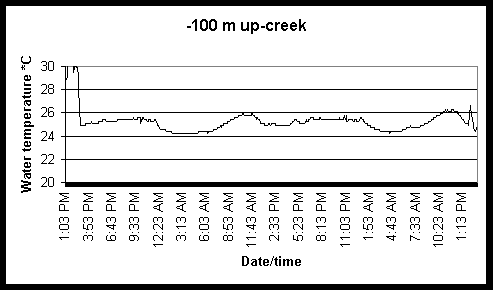
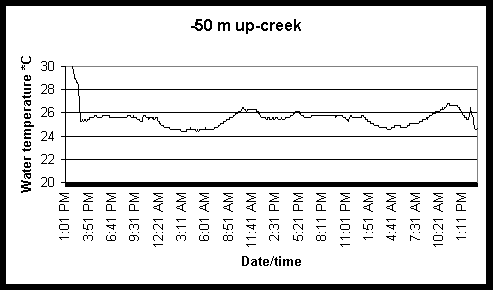
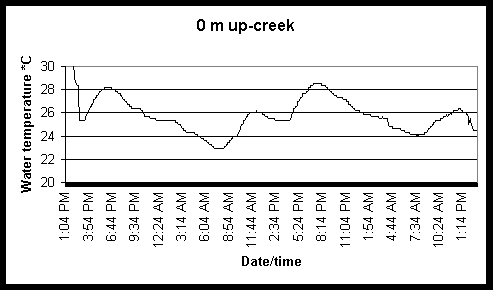

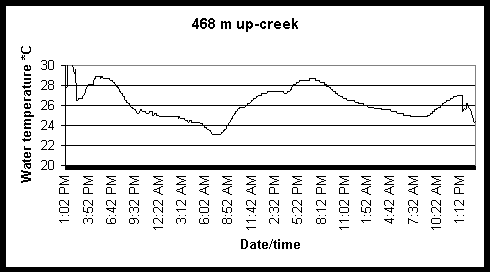
Figure 6. Diel and tidal changes in water temperature at five locations along the marsh gradient during the week of August 2-6 1999.
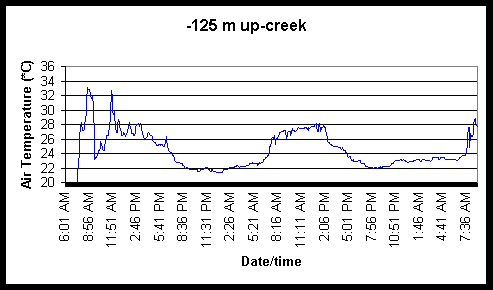
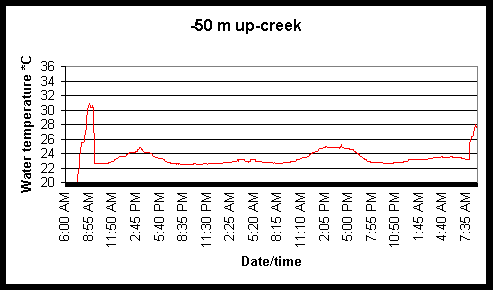
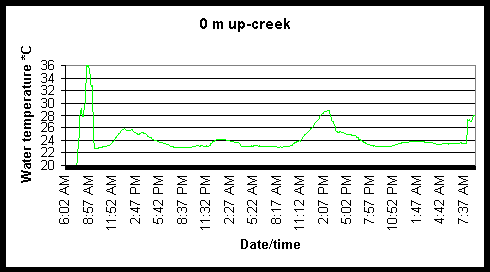
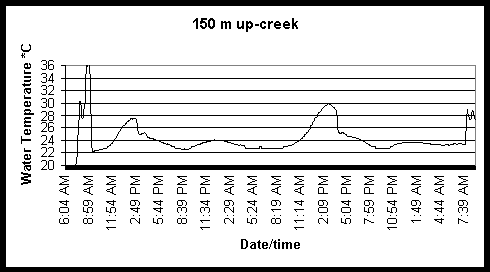

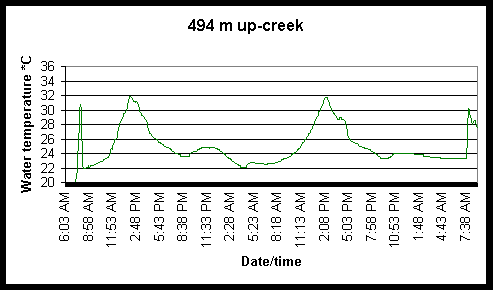
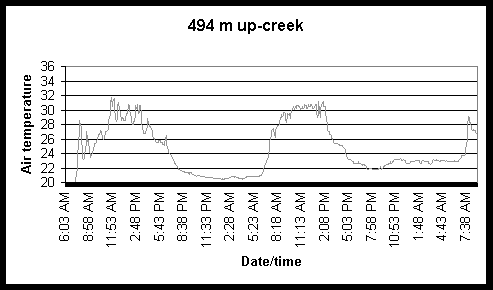
Figure 7. Diel and tidal changes in water temperature at five locations, and air temperature at two locations, along the marsh gradient during the week of August 23-27 1999.

Figure 8. Schematic illustrating the standardized seine sampling methodology used during this study. Prior to each sample event a pair of adjacent 5-m sections of the creek were randomly selected within each strata. A) The block nets, clotheslines, pull-ropes and support poles were set in place at high tide. The pull-ropes were attached to block nets stored by the side of the creek and were stretched across the creek to the opposite side. At low tide all three block nets were simultaneously deployed by pulling them across the creek.
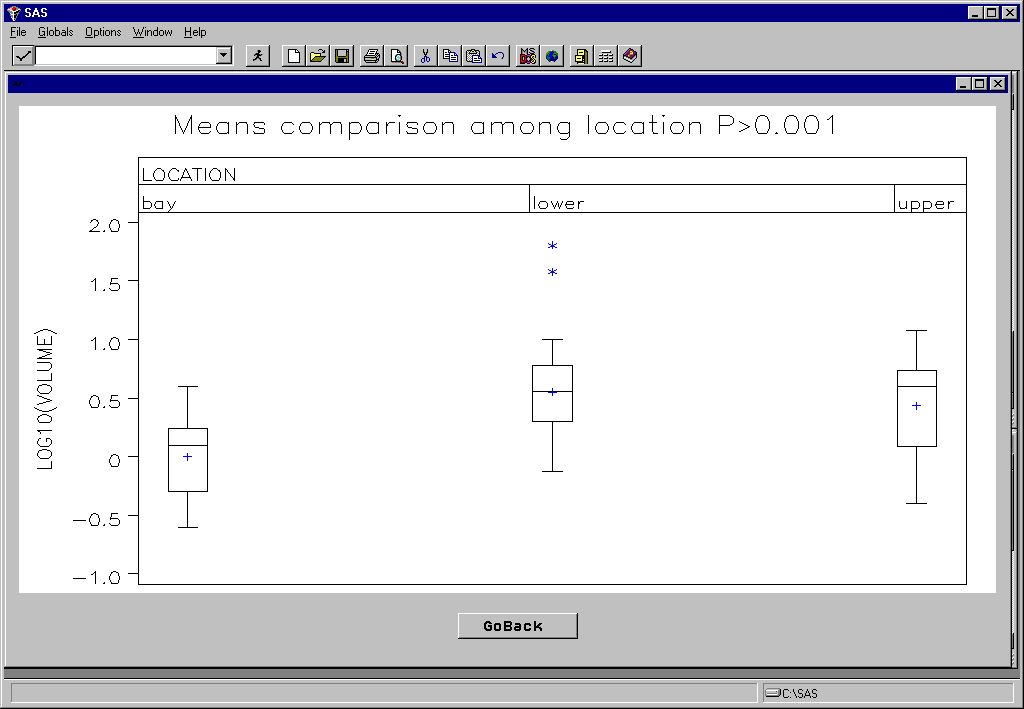
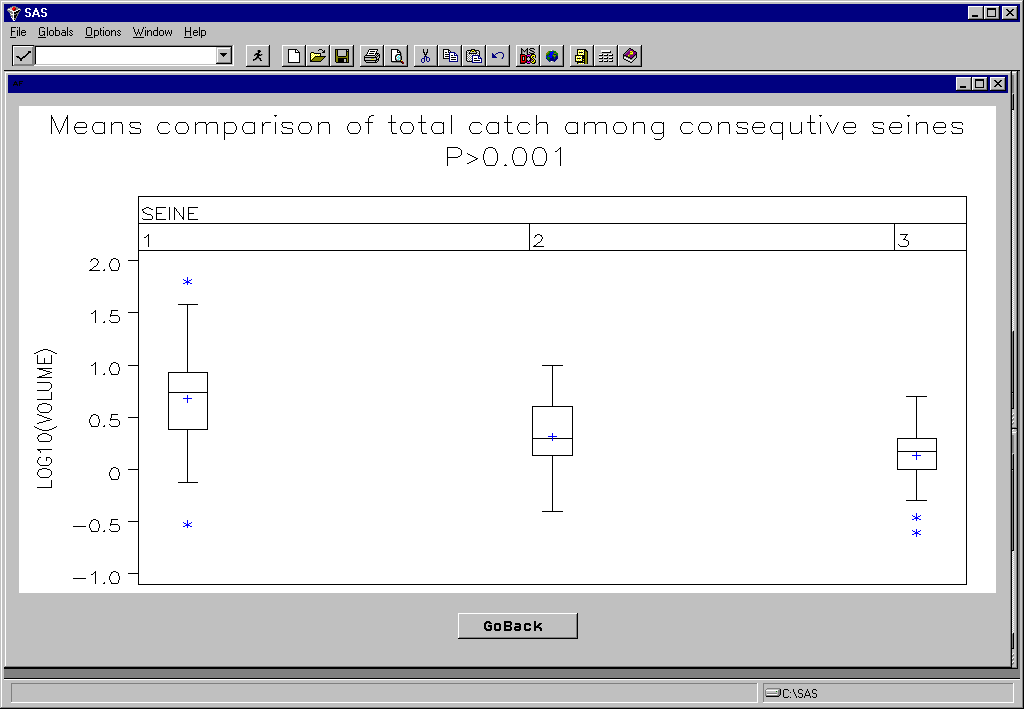
Figure 9. Highly significant differences among consecutive seine number and among strata (location) main effects on total volume of the catch (as measured in the field) were found.
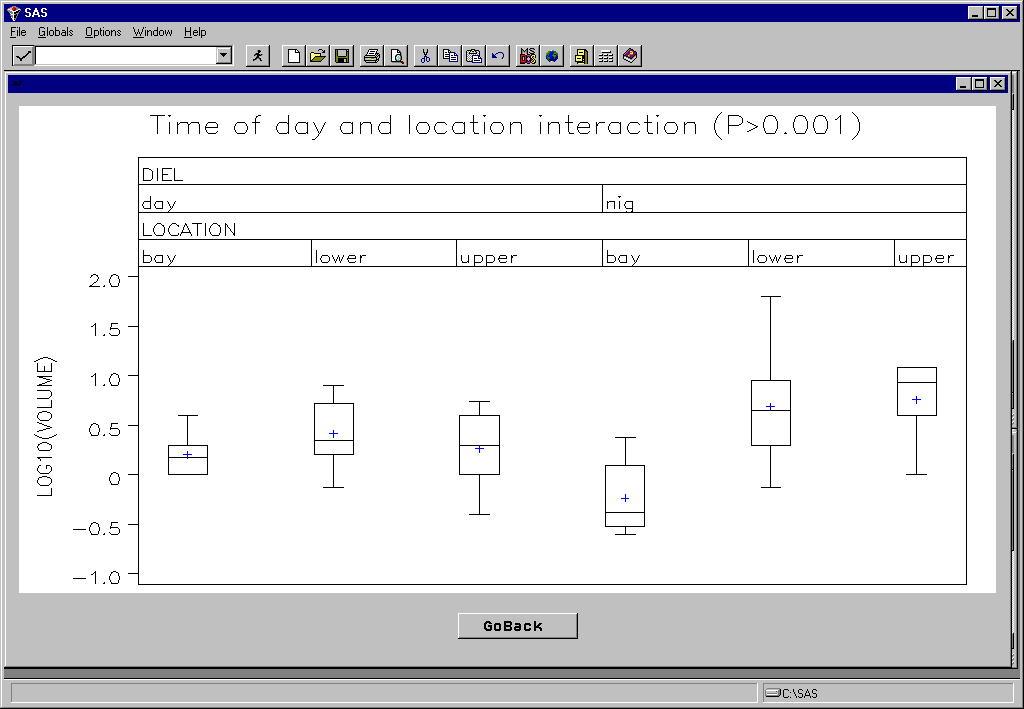
Figure 10. A highly significant interaction between diel (day/night) and strata (location) main effects on total seine catch (as measured in the field) was observed.

Figure 11. A non-significant trend suggesting an interaction between seine type (experimental versus conventional) and consecutive seine number was observed in which a strong reduction in total catch volume was observed for the experimental seines (B and C), but not for the conventional control seines (A).
Return to :| TOP |
This page was last modified on July 20, 2001
Copyright (c) 1999 by Rodney Rountree. All rights reserved
Navigate to main estuarine pages: [ | merp | weirmeth ]
Navigate to main pages: [ Diets | Photos | Estuarine | FADs | Soniferous | CV | Home Page ]